Helicobacter pylori Test– SUBT-D1 Detector & C14 Urea Breath Test Kits
Helicobacter pylori (H. pylori) is a bacterium infecting the stomach lining, responsible for a large percentage of gastric and duodenal ulcers and even linked to stomach cancer. In fact, roughly 50% of the world’s population is estimated to carry H. pylori. Early detection and treatment of H. pylori are critical to prevent serious conditions like peptic ulcers and gastric cancer. For clinics and distributors in Belgium seeking reliable Helicobacter pylori test solutions, urea breath testing offers a fast, accurate, and non-invasive option. Global clinical guidelines endorse the urea breath test (UBT) as the best recommended non-invasive diagnostic method for H. pylori in a “test-and-Gentaur now provides access to state-of-the-art H. pylori breath test kits and analyzers – specifically the Celis Biotech SUBT-D1 detector and C14 UBT kits – enabling distributors and clinics in Belgium to perform Helicobacter pylori breath tests with confidence.
Why Choose a Helicobacter pylori Urea Breath Test ?
H. pylori breath testing has become a preferred diagnostic tool for several key reasons :
- Non-Invasive & Patient-Friendly : Unlike endoscopy or biopsies, a urea breath test is as simple as having the patient swallow a capsule and exhale into a collection device. No special preparation is needed for the patient, and the procedure is painless and quick. This ease and comfort mean high patient compliance and broad acceptance of the test.
- High Accuracy : Urea breath tests offer excellent accuracy, with clinical sensitivity and specificity often above 95%. The SUBT-D1 detector, for instance, is highly reliable, boasting >95% sensitivity and specificity for H. pylori detection. This level of performance rivals or exceeds other non-invasive tests (like stool antigen) and far outperforms serology, which cannot distinguish active infection.
- Rapid Results : The entire breath test procedure can be completed in one short clinic visit (typically around 15–20 minutes for ingestion and sample collection, plus a few minutes for analysis). This means patients can get results on the same visit, allowing for immediate consultation and treatment decisions.
- Ideal for Test-and-Treat Strategy : Because of its accuracy and convenience, UBT is ideal for the “test-and-treat” strategy recommended by gastroenterology guidelines. A positive result indicates active infection that can be treated, and the test can easily be repeated after therapy to confirm eradication.
- Safe, Low Radiation Dose : The C14 UBT uses a minute amount of radioactive carbon-14, but the dose is extremely low (on the order of micro-Sieverts) – considered safe for adults and even children, with no special radiation precautions required in routine use. This makes the test safe for widespread clinical use.
In summary, a Helicobacter pylori breath test offers a combination of accuracy, patient comfort, and clinical speed that is hard to beat. Below, we introduce the two key components of Gentaur’s H. pylori breath testing solution in Belgium: the SUBT-D1 urea breath test detector and the C14 urea breath test kits.
SUBT-D1 Helicobacter Pylori Detector – Features and Benefits
The SUBT-D1 is a professional Helicobacter pylori detector developed by Celis Biotech. It is a 14C urea breath test analyzer designed for both initial H. pylori diagnosis and post-treatment follow-up testing. This device is essentially the analytical unit that reads patients’ breath samples after they have taken the urea capsule. Below are key features and benefits of the SUBT-D1 detector :
- Proven 14C Urea Breath Test Technology : The SUBT-D1 uses the gold-standard 14C UBT method. The patient simply swallows a capsule containing a small amount of ^14C-labeled urea. If H. pylori is present in the stomach, the bacterium’s urease enzyme metabolizes the urea into ^14CO_2 and ammonia. This ^14CO_2 is absorbed into the bloodstream and carried to the lungs, where it is exhaled after about 15 minutes. The SUBT-D1 system captures the exhaled breath using a special collection card, and then measures the ^14C in the breath sample to detect infection.
- High Accuracy and Reliability : As mentioned, the device provides very accurate results, with sensitivity and specificity greater than 95% in clinical evaluations. It’s a reliable and smart system that minimizes false results. Uniquely, if the initial reading is in a borderline range, the SUBT-D1 will automatically perform a second measurement to confirm the result. This ensures that even borderline cases are correctly classified, improving diagnostic confidence.
- Non-Invasive & Patient Safe : No blood draws, no endoscopy – the test is entirely non-invasive. There’s no dietary or medication preparation required in most cases (patients don’t even need to fast for long periods). The low-dose ^14C capsule is safe and well-tolerated, making the test suitable for a wide range of patients.
- Fast and Efficient : The SUBT-D1 provides on-the-spot results. Once the patient has breathed into the collection card, the card is inserted into the machine, and the device analyzes the sample within minutes. This rapid turnaround allows doctors to discuss treatment in the same visit if the result is positive.
- User-Friendly Design : Celis Biotech has designed the SUBT-D1 with ease of use in mind. The system is plug-and-play, with simple installation and minimal maintenance required. It features a clear digital display and user-friendly software, so clinic staff can operate it with minimal training. Calibration and controls are straightforward, ensuring consistent performance.
- Portable & Compact : The device has a compact, portable design, roughly the size of a small desktop analyzer. This makes it easy to fit into any clinic or laboratory setting without requiring much space. It can even be transported between locations if needed for mobile testing events or multi-site use.
- Clinical Versatility : The SUBT-D1 is suitable for both primary diagnosis of H. pylori in new patients and for confirming eradication after treatment (typically done 4-6 weeks post-therapy). Having this single device allows clinics to cover the full patient pathway – from initial test-and-treat through follow-up testing – with one reliable tool.
The SUBT-D1 Helicobacter pylori detector is a compact 14C urea breath analyzer with a clear display and simple controls. Its user-friendly interface and plug-and-play setup allow quick implementation in any clinic or lab. This portable device enables on-site H. pylori testing, so patients can be diagnosed within a single appointment, improving workflow and patient satisfaction.
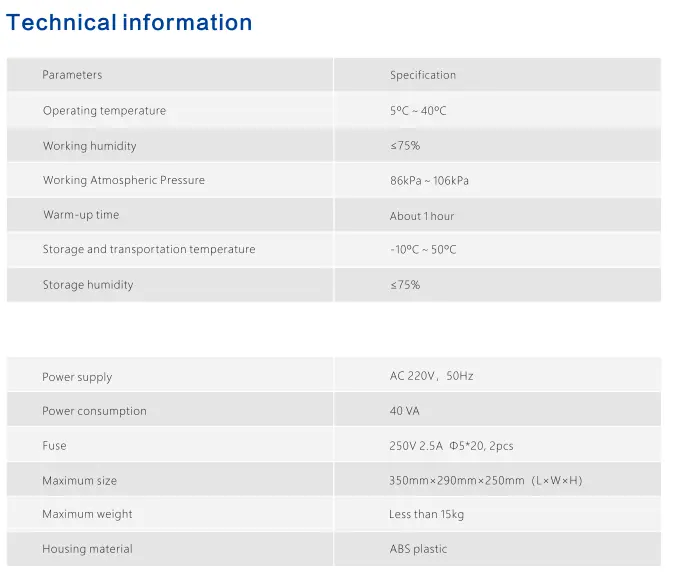
How Does the SUBT-D1 Detector Work ?
Using the SUBT-D1 in practice is straightforward. After a patient ingests the ^14C-urea capsule (usually on an empty stomach for optimal results), there is a 15-minute waiting period. During this time, if H. pylori bacteria are present in the stomach, they break down the urea and ^14CO_2 is absorbed into the bloodstream. The patient then blows into a specialized breath collection card through an attached mouthpiece. The blowing is done for a few minutes until an indicator on the card changes color (from blue to white), ensuring an adequate breath sample is captured. The key steps of the testing procedure are :
- Ingest the Urea Capsule : The patient swallows a ^14C-urea capsule with water. This is done on an empty stomach (no food for at least 2 hours prior) for best results.
- Wait for 15 Minutes : The patient remains seated and still for about 15 minutes to allow the urea to metabolize
- Collect the Breath Sample : The technician provides a breath collection card (with an attached disposable mouthpiece). The patient exhales into the card steadily. They can breathe normally in between exhalations but should avoid inhaling through the card. Blowing continues for up to 3 minutes, or until the card’s indicator spot turns from blue to white, showing the sample is collected.
- Analyze the Sample : The used card is then inserted into the SUBT-D1 analyzer. The device automatically measures the radio-labeled CO_2 trapped in the card and computes the result. Within minutes, the SUBT-D1 displays a quantitative reading that indicates whether H. pylori is present. The medical staff can then report the result to the patient.
(The breath test kit and its usage are discussed in more detail in the next section. In essence, the SUBT-D1 detector works hand-in-hand with the C14 UBT kits: patients take the kit’s capsule and blow into the kit’s card, and the SUBT-D1 reads that card to give the result.)


Interpreting SUBT-D1 Test Results
The SUBT-D1 provides a clear, quantified result based on the amount of ^14CO_2 detected in the breath sample. The result is typically given in terms of DPM (disintegrations per minute), a measure of the radioactivity from the ^14C. The device uses preset cut-off values to interpret the DPM reading :
- If the reading is ≤ 99 DPM, it is considered Negative (no active H. pylori infection).
- If the reading is just above the cutoff (around 100–149 DPM), the device will automatically repeat the measurement to double-check. If it remains elevated on repeat, the result is considered Positive (borderline).
- Higher readings correspond to clearly positive results, and the SUBT-D1 may categorize them with incremental “+” signs to indicate relative infection load. For example, a result of 150–499 DPM is reported as Positive (+), 500–1499 DPM as Positive (++), 1500–2499 DPM as Positive (+++), and anything above ~2500 DPM as Positive (++++). These gradations can give clinicians a sense of how strong the urease activity (and by proxy, bacterial load) is in the patient’s stomach.
In practice, any confirmed reading above the cutoff (~99 DPM) means an active H. pylori infection is present. The nuanced scaling of +/++/+++ is optional information, but it underscores the clinical reliability of the device – even low-level infections can be caught (just above the cutoff), and very high readings are clearly distinguished. The automatic re-test feature around the borderline range adds an extra layer of confidence that no false negatives slip through. Overall, the interpretation is straightforward: “Negative” means no infection, and any “Positive” means H. pylori is detected, prompting treatment or further evaluation.

C14 Urea Breath Test Kits – Simple, Accurate H. pylori Testing
While the SUBT-D1 is the analyzer instrument, the C14 Urea Breath Test kits are the consumable testing materials used for each patient. Each kit is a single-patient test that comes with two main components: a urea capsule and a breath collection card. The kit provides everything needed to perform one H. pylori breath test on a patient. Gentaur offers these kits in bulk quantities to clinics and laboratories. Key features of the C14 UBT kits :
- Complete Sampling Package : Each kit is a “package of [a] collection card and capsule” designed specifically for the ^14C Urea Breath Test. The ^14C-urea capsule (often 37 kBq activity, a very low and safe dose) is swallowed by the patient. The breath collection card is a small, chemically-treated card that absorbs the ^14CO_2 from the exhaled breath. Together, these allow clinicians to easily administer the test and collect a sample for analysis.
- Easy to Use : The procedure for using the kit is straightforward, as outlined earlier. The simplicity of the steps (swallow capsule, wait, breathe into card) means that minimal training is required for staff. Patients also find the process easy – there’s no discomfort aside from possibly a bland taste from the capsule. The entire process takes under 20 minutes, causing minimal disruption to the clinic workflow.
- Non-Invasive and Patient-Friendly : Just like the SUBT-D1 device, the kit’s usage is non-invasive. Patients generally appreciate that they “just have to breathe” for this test, as opposed to undergoing endoscopy or providing blood/stool samples. This makes the C14 UBT kit an excellent choice for pediatric patients or those averse to invasive procedures.
- Highly Accurate Results : When used with the SUBT-D1 analyzer, the kits yield highly accurate results. The principle of detecting active urease activity in the stomach means the test is specific to active H. pylori infection. There’s no cross-reactivity or confusion with past infections (as can happen with antibody tests). If H. pylori is present, the kit will capture the evidence in the breath. If H. pylori is absent or already eradicated, the test will remain negative. This makes the kits ideal for post-treatment confirmation of cure – a negative breath test after therapy is a reliable indicator that H. pylori is gone.
- Stable and Convenient : The breath collection cards in the kit are designed to safely trap the ^14CO_2 and remain stable for analysis. In practice, once a patient blows into the card, it can be inserted into the SUBT-D1 immediately. (If needed, the card could even be stored or transported short-term before reading, since the absorbed ^14C doesn’t degrade quickly – although on-site immediate reading is the norm.) The kits come with simple instructions and are individually packed, ensuring hygiene and convenience.
Using the C14 UBT kit – Step by Step : (recap)
To highlight the simplicity, here’s a recap of how a typical H. pylori breath test kit is used in a clinical setting :
- Capsule Ingestion : The patient swallows the provided ^14C-urea capsule with water (fasting for ~2 hours beforehand).
- Waiting Period : The patient waits for 15 minutes calmly, allowing the urea to interact with any H. pylori in the stomach.
Breath Collection: The healthcare provider opens the breath collection card from its packaging and attaches the disposable mouthpiece. The patient blows into the card steadily. They continue exhaling (with normal breaths in between) for up to 3 minutes, or until the card’s indicator spot fully changes color (blue to white), indicating the sample is collected.
- Seal & Submit : Once done, the card is given back to the medical staff and typically inserted into the SUBT-D1 analyzer right away for measurement. (Each card usually has a protective holder or sleeve to avoid contamination and contain the sample.)
vfg
The breath test kits are designed to be used at point-of-care – right in the doctor’s office, clinic, or lab. There’s no need to send samples out to a reference lab; the breath sample is analyzed on the spot with the SUBT-D1. This combination of C14 UBT kit + SUBT-D1 detector essentially creates a mini in-house diagnostic system for H. pylori that any clinic in Belgium can now set up via Gentaur.
Bulk Packaging : For bulk purchasers (such as hospitals or distributors), the kits are supplied in larger cartons. Each carton contains 400 individual test kits and weighs about 12 kg. This bulk packaging is convenient for inventory management and shipping. Clinics can store the kits at room temperature (no special storage conditions required aside from usual room temp and dryness) and have them on hand whenever they need to test patients.
Now, let’s get into the specifics that distributors and procurement managers will care about – pricing, quantities, and shipping details for Belgium.
Product Specifications, Pricing & Shipping (Brussels, Belgium)
Gentaur offers competitive pricing for bulk orders of the SUBT-D1 H. pylori detector and C14 urea breath test kits, with convenient shipping to Brussels and all of Belgium. Below are the pricing tiers, packaging specs, and shipping costs for each product :
SUBT-D1 Detector – Pricing & Shipping
- Pricing (Bulk Order Discounts) : For an order of 5 units of the SUBT-D1 detector, the price is USD $3,200 per unit. For larger orders of 10 units or more, the price drops to USD $3,000 per unit. These tiered prices allow significant savings for distributors or larger clinics purchasing multiple devices. (For quantities between 5 and 10, please contact Gentaur for a quote – we can accommodate and provide competitive pricing.)
- Product Specs : Each SUBT-D1 unit is a standalone analyzer device. It comes with its power supply and a user manual. The physical dimensions of the device are approximately 45 × 40 × 37 cm per unit, and it weighs about 12 kg per unit. Its compact size means shipping is not cumbersome, but the weight (~12 kg) reflects its solid build and internal detection components.
- Packing & Shipping : The detectors are securely packed for international shipping. Shipping to Brussels, Belgium is estimated at USD $200 per unit. This cost covers air freight or expedited delivery to ensure the delicate electronics arrive safely and promptly. Gentaur handles the customs clearance and logistics – customers in Belgium will receive the device delivered to their door or warehouse. Typical transit time is quick, and we ensure all necessary documentation is provided for a smooth import process.
C14 Urea Breath Test Kits – Pricing & Shipping
- Pricing (Bulk Quantities) : The breath test kits are sold in bulk packs. For a minimum order of 400 kits (which is one carton), the price is USD $7 per kit. Larger volume discounts are available: if you order 4,000 kits, the price is USD $6.50 per kit; for orders of 10,000 kits or more, the price goes down to USD $6 per kit. These price breaks make the C14 UBT kits highly affordable per test, especially for hospitals or national distributors ordering in large volumes.
- Packaging Details : 400 kits per carton. Each carton weighs about 12 kg and contains 400 individually packaged test kits. The cartons are sturdy and suitable for storage or shipping. They are labeled with batch details and have a long shelf-life (the kits are stable for many months).
- Shipping to Brussels : Gentaur ships the kits to Belgium with care. The estimated shipping cost is USD $180 per carton (400 kits). We use reliable couriers/freight services to ensure the kits arrive in good condition. Given the relatively lightweight nature of each carton (~12 kg), bulk shipments of multiple cartons are manageable. We can arrange pallet shipments for very large orders (e.g., 10,000 kits would be 25 cartons) at optimized freight rates. As always, Gentaur takes care of customs paperwork, and our clients in Brussels or elsewhere in Belgium will get the kits delivered hassle-free to their location.
dcgb
Note on Ordering & Availability : Both the SUBT-D1 devices and the C14 UBT kits are kept in stock or have short lead times thanks to Celis Biotech’s efficient manufacturing. When you place an order with Gentaur, we will provide an expected delivery timeline. Small trial orders can be arranged if you wish to evaluate the product, and thereafter, bulk orders can be fulfilled quickly to meet your demand.

Buy H. pylori Detector & Test Kits Online via Gentaur in Belgium
If you are a distributor or a clinic in Belgium looking for a reliable C14 UBT kit distributor or source for H. pylori testing equipment, Gentaur is here to serve you. We are proud to partner with Celis Biotech as a distributor of the SUBT-D1 detector and C14 urea breath test kits, bringing these advanced products to the Belgian market. You can easily buy the H. pylori detector online via Gentaur’s website or by contacting our sales team for a personalized quote.
Ready to order ? Contact Gentaur today to equip your laboratory or medical practice with the SUBT-D1 detector and C14 UBT kits. We offer prompt communication and will guide you through the purchasing process – from pricing inquiries to shipping arrangements – ensuring your order arrives safely in Brussels (or anywhere in Europe you require). With our competitive pricing and dedicated customer support, Gentaur makes it simple to integrate H. pylori breath testing into your services.
Don’t miss out on the opportunity to enhance your diagnostic capabilities with this clinically proven, non-invasive H. pylori test. Whether you run a gastroenterology clinic that needs a better way to diagnose ulcers, or you’re a distributor aiming to supply hospitals with quality breath test kits, the combination of the SUBT-D1 detector and C14 UBT kits is an ideal solution. Order now through Gentaur and provide your clients and patients in Belgium with the accuracy, convenience, and peace of mind that come with modern H. pylori breath testing. Contact us via phone or email (or visit our website) to get started – and take the first step toward easier H. pylori detection and improved gastrointestinal care!